-
ISO 13485 QUALITY SYSTEMS
-
ISO 13485 PROCEDURE BUNDLES
-
ISO 13485 QUALITY SYSTEM PROCEDURES
-
VALIDATION PROTOCOLS
-
CONTRACTS - AGREEMENTS
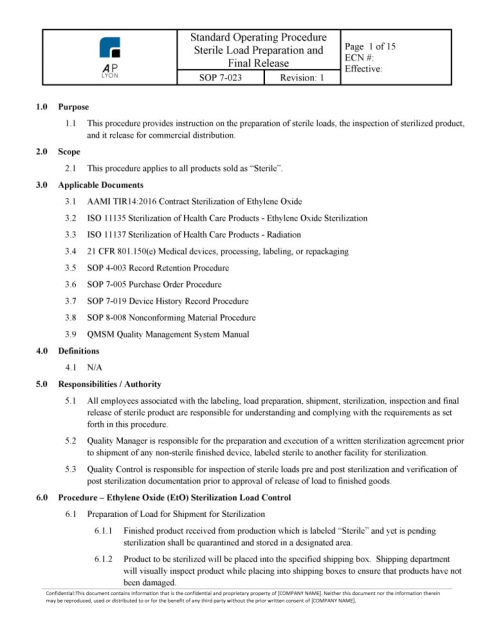
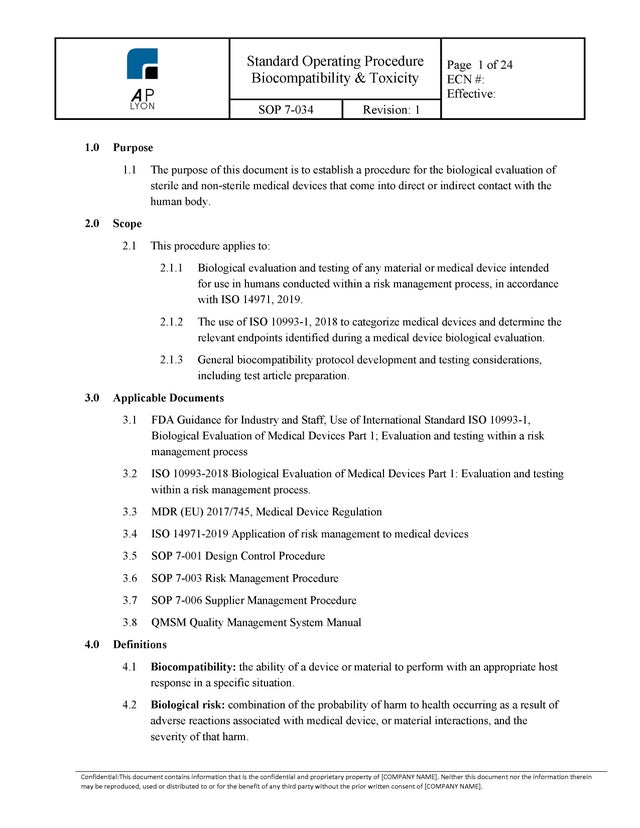
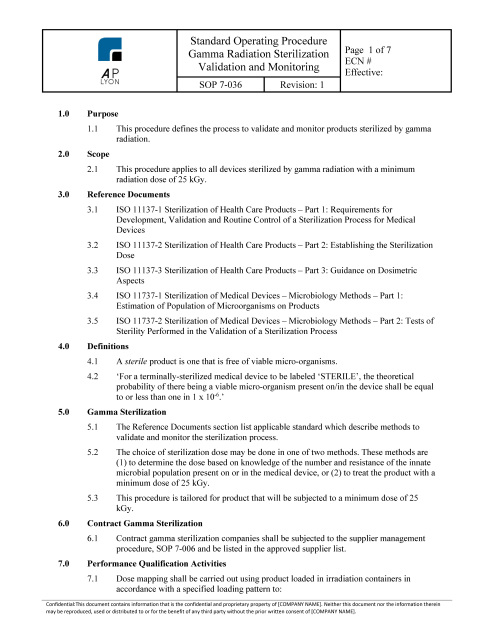
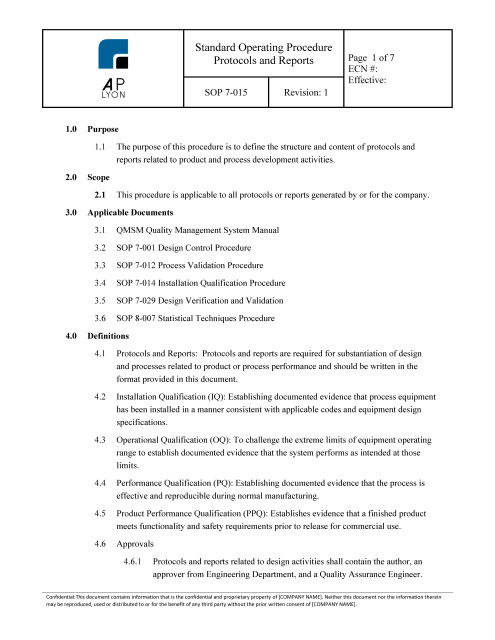
ISO 13485 Quality Management System Procedures | FDA QSR Compliant
ISO 13485 QUALITY SYSTEM PROCEDURES

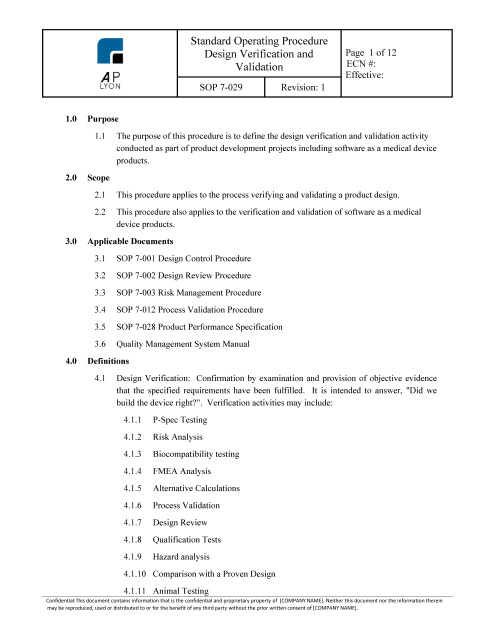
Clinical Evaluation Procedure
$149.00
$149.00
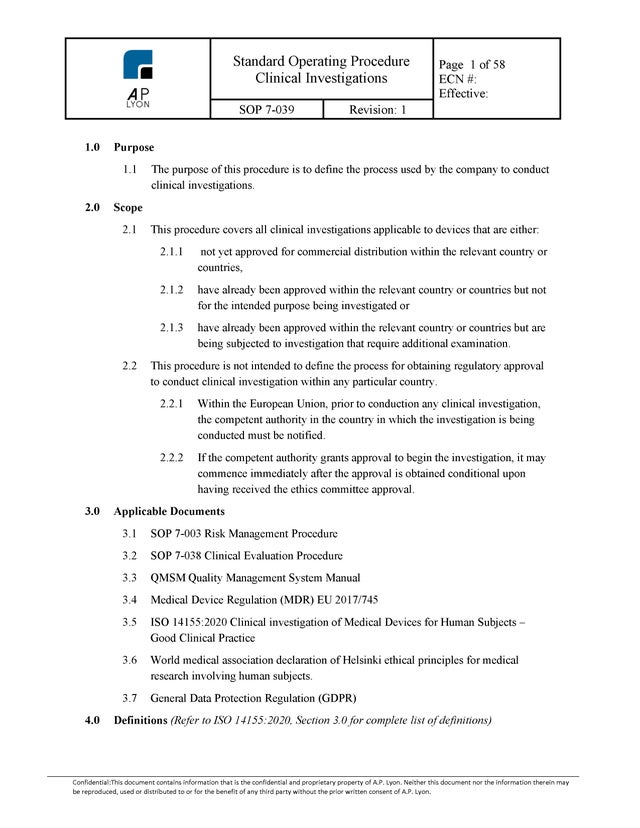
Clinical Investigation Procedure
$199.00
$199.00

Design Control Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00
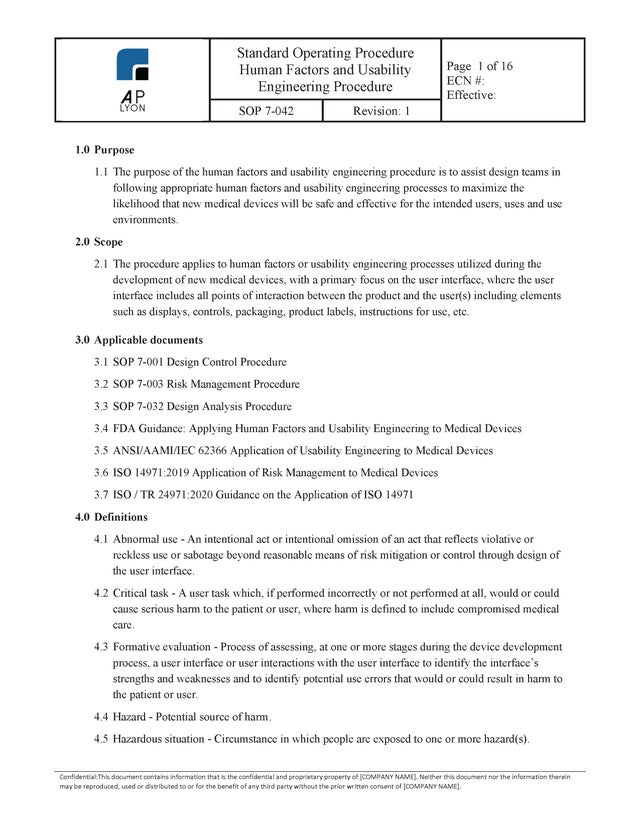
Human Factors and Usability Engineering Procedure
$149.00
$149.00

Design Review Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Design Transfer Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00
Risk Management Procedure
$149.00
$149.00

Design Risk Management Procedure
$139.00
$139.00

Process Risk Management Procedure
$139.00
$139.00

Design Analysis Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Complaint Handling Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Document Control Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Software Validation Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00
Unique Device Identification Procedure
$149.00
$149.00

Management Review Procedure | ISO 13485 | FDA QSR Compliant
$119.00
$119.00

Supplier Management Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00
Environmental Controls Procedure | ISO 13485 | FDA QSR Compliant
$119.00
$119.00
Training Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Identification Procedure | ISO 13485 | FDA QSR Compliant
$99.00
$99.00
Lot Inspection Procedure | ISO 13485 | FDA QSR Compliant
$119.00
$119.00

Technology Transfer Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Internal Audit Procedure | ISO 13485 | FDA QSR Compliant
$99.00
$99.00

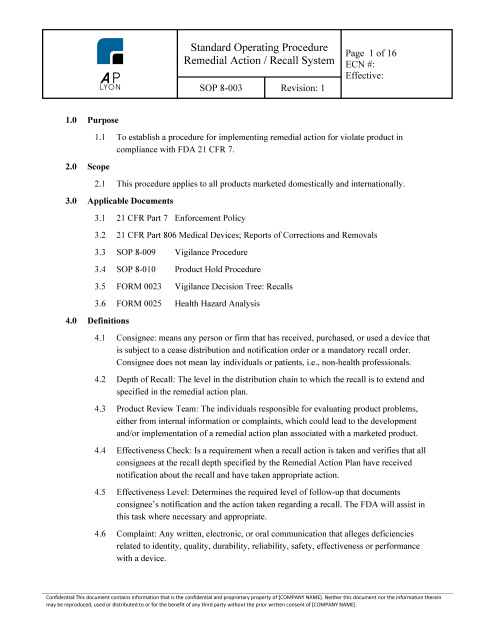
Medical Device Report MDR Procedure | FDA QSR Compliant
$149.00
$149.00

Statistical Techniques Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Nonconforming Material Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00
Product Hold Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

First Article Inspection Procedure
$49.00
$49.00

Facility Inspection Procedure
$149.00
$149.00

Vigilance Procedure
$149.00
$149.00

Post Market Surveillance Procedure
$149.00
$149.00
ISO 13485 Label Review and Approval Procedure
$139.00
$139.00

ISO 13485 Contract Review Procedure
$49.00
$49.00

CE Marking Procedure
$149.00
$149.00

Preventive Maintenance Procedure
$49.00
$49.00

Respiratory Protection Procedure
$149.00
$149.00

Biohazard Procedure
$149.00
$149.00

Lockout - Tagout Procedure
$149.00
$149.00
Injection Mold Validation Procedure
$149.00
$149.00
Line Clearance Procedure
$49.00
$49.00

ISO 13485 Quality Management System Manual
$139.00
$139.00

Software as a Medical Device (SaMD) Development Procedure
$149.00
$149.00

Software Clinical Evaluation Procedure
$149.00
$149.00

Cleanroom Design and Validation Procedure
$149.00
$149.00

Customer Requirements Validation Procedure
$149.00
$149.00

Device History Record Procedure
$149.00
$149.00

Periodic Safety Update Report (PSUR) Procedure
$139.00
$139.00

Failure Investigation Procedure
$149.00
$149.00

Purchase Order Procedure | ISO 13485
$99.00
$99.00
Record Retention Procedure
$49.00
$49.00

Medical Device Product Performance Specification Procedure
$149.00
$149.00

Medical Device Process Validation Procedure
$149.00
$149.00
Medical Device Clinical Investigation Plan
$49.00
$49.00
Medical Device Clinical Investigation Report
$49.00
$49.00