-
ISO 13485 QUALITY SYSTEMS
-
ISO 13485 PROCEDURE BUNDLES
-
ISO 13485 QUALITY SYSTEM PROCEDURES
-
VALIDATION PROTOCOLS
-
CONTRACTS - AGREEMENTS
ISO 13485 Quality Management Systems | Section 8 | Measurement Analysis Improvement
|
|
ISO 13485 MEASUREMENT ANALYSIS IMPROVEMENT PROCEDURES

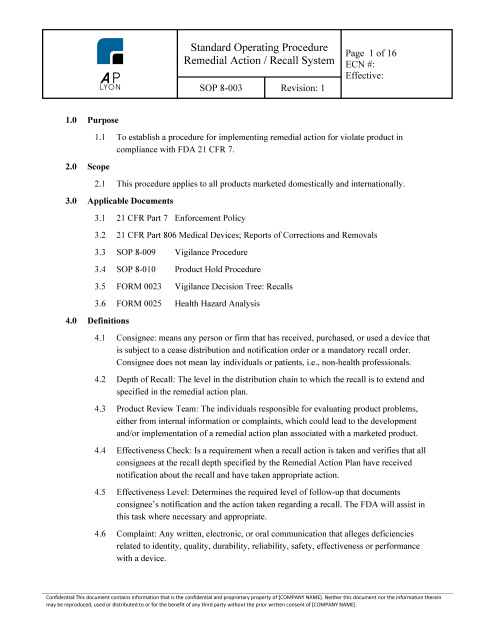
Customer Requirements Validation Procedure
$149.00
$149.00

Complaint Handling Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Internal Audit Procedure | ISO 13485 | FDA QSR Compliant
$99.00
$99.00

Medical Device Report MDR Procedure | FDA QSR Compliant
$149.00
$149.00

Statistical Techniques Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Nonconforming Material Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00
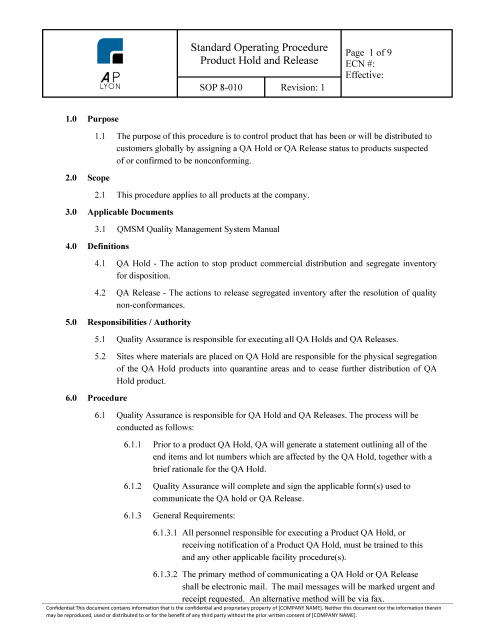
Product Hold Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Vigilance Procedure
$149.00
$149.00

Post Market Surveillance Procedure
$149.00
$149.00

Periodic Safety Update Report (PSUR) Procedure
$139.00
$139.00

Failure Investigation Procedure
$149.00
$149.00

Post Market Clinical Follow Up Plan
$49.00
$49.00

Post Market Clinical Follow-up (PMCF) Evaluation Report
$49.00
$49.00