-
ISO 13485 QUALITY SYSTEMS
-
ISO 13485 PROCEDURE BUNDLES
-
ISO 13485 QUALITY SYSTEM PROCEDURES
-
VALIDATION PROTOCOLS
-
CONTRACTS - AGREEMENTS
Validation Protocols for Medical Device Manufacturers
VALIDATION PROTOCOLS
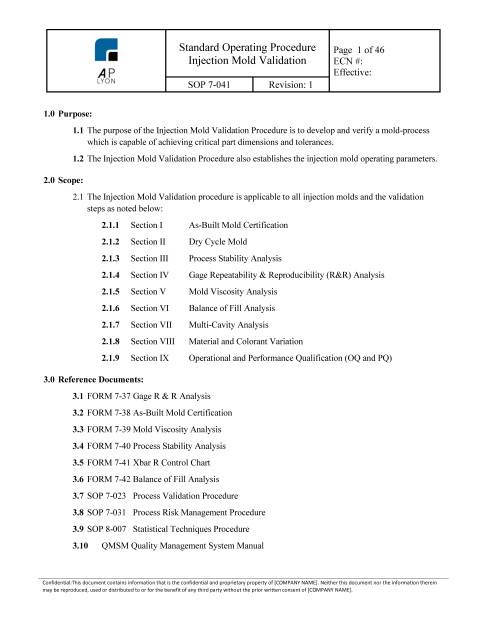
Injection Mold Validation Procedure
$149.00
$149.00

Ethylene Oxide (EO) Product Adoption Protocol
$149.00
$149.00

Software Validation Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Final Cleaning Orthopedic Implants
$149.00
$149.00

Accelerated Aging Study
$149.00
$149.00

Customer Requirements Validation Procedure
$149.00
$149.00

Medical Device Software Development Plan
$119.00
$119.00
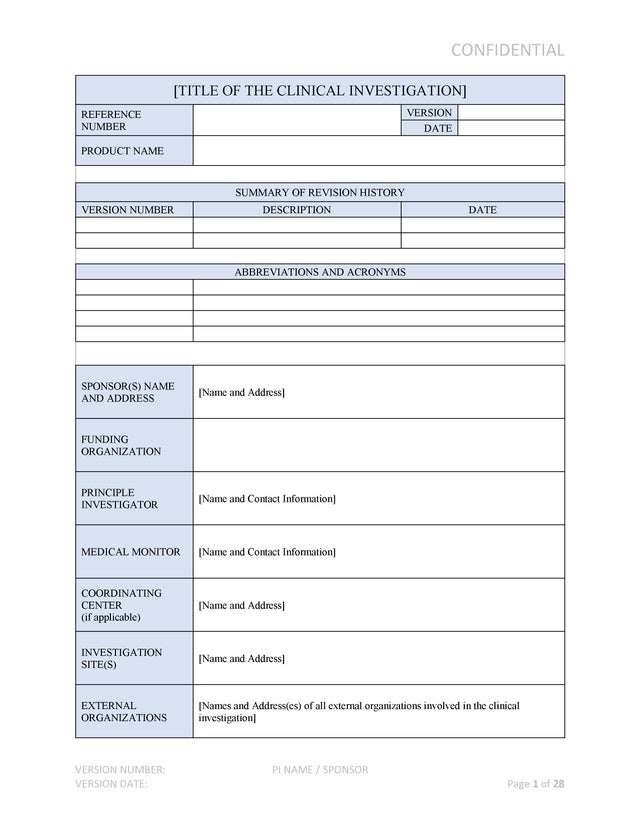
Medical Device Clinical Investigation Plan
$49.00
$49.00
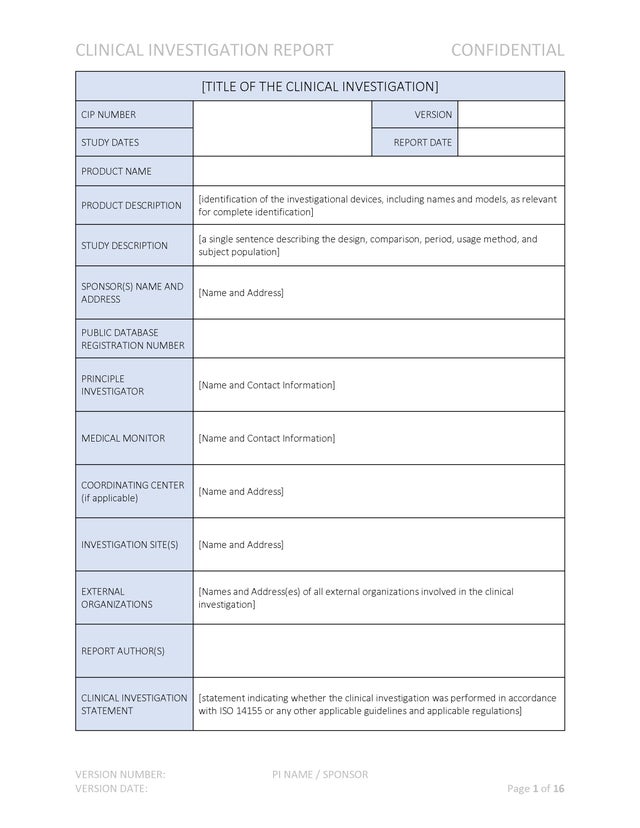
Medical Device Clinical Investigation Report
$49.00
$49.00









