- STORE
- >
- ISO 13485 QUALITY SYSTEM PROCEDURES
- >
- ISO 13485 PRODUCT REALIZATION PROCEDURES
- >
- ISO 13485 DESIGN DEVELOPMENT PROCEDURES
- >
- Software Validation Procedure | ISO 13485 | FDA QSR Compliant
Software Validation Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00
Unavailable
per item
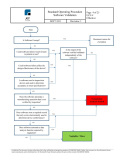
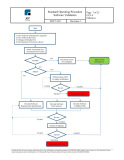
The Software Validation Procedure governs computer systems and medical device software used in medical device development, production and QA activities. The procedure includes a detailed validation protocol with step by step instruction for conducting the validation and generating a validation report.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
SKU:
SOP 7-033
ISO 13485 Software Validation ProcedureThe Software Validation Procedure governs computer systems and medical device software used in medical device development, production and QA activities. The protocol, included with the procedure, can be applied to the validation of computer systems, software used in medical device development, production, testing and inspection activities, and more to ensure each has been validated for their intended use.
ISO 13485 Software Validation Procedure OverviewThe Software Validation Procedure uses a risk based approach for conducting software validations. The validation protocol included with the procedure provides step by step instruction through the entire validation process. The procedure also provides instruction on the content and structure of validation reports.
Software Validation Procedure ComplianceThe Software Validation Procedure is FDA 21 CFR Part 820 and ISO 13485:2016 compliant.
To learn more about our Software Validation Procedure contact us at [email protected]
RELATED LINKS |
|
Customers also viewed
Medical Device Software Procedure Bundle
$449.00
The Medical Device Software Procedure Bundle includes procedures related to development of software products, validation of software, software clinical evaluation, and how to apply human factors and usability engineering to the medical device development process. Also includes Medical Device Software Development Plan.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
Medical Device Software Development Plan
$119.00
The Medical Device Software Development Plan provides detailed instruction on the creation of a software development plan that is harmonized with the requirements of IEC 62304 and FDA guidance.
- IEC 62304:2015 Compliant
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Instructive Document
- Instant Download - Digital Content
Software as a Medical Device (SaMD) Development Procedure
$149.00
The SaMD Development Procedure governs the planning and realization of Software as a Medical Device (SaMD) product lifecycle processes. The procedure provides instruction and guidance throughout the software lifecycle and establishes critical linkage to QMS and regulatory requirements.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- IEC 62304:2015 Compliant
- MS Word Format
- Digital Content - Instant Download
Software Clinical Evaluation Procedure
$149.00
The Software Clinical Evaluation Procedure governs the clinical evaluation activities, including the assessment and analysis of the clinical safety, effectiveness and performance, of the Software.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MDR (EU) 2017/745 Compliant
- MS Word Format
- Digital Content - Instant Download