-
ISO 13485 QUALITY SYSTEMS
-
ISO 13485 PROCEDURE BUNDLES
-
ISO 13485 QUALITY SYSTEM PROCEDURES
-
VALIDATION PROTOCOLS
-
CONTRACTS - AGREEMENTS
ISO 13485 Quality Management Systems | Section 7 | Product Realization | FDA QSR Compliant
ISO 13485 PRODUCT REALIZATION PROCEDURES
Risk Management Procedure
$149.00
$149.00

Design Risk Management Procedure
$139.00
$139.00

Process Risk Management Procedure
$139.00
$139.00

Design Control Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00
Human Factors and Usability Engineering Procedure
$149.00
$149.00

Clinical Evaluation Procedure
$149.00
$149.00
Clinical Investigation Procedure
$199.00
$199.00

Design Review Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Design Analysis Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Design Transfer Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Supplier Management Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Identification Procedure | ISO 13485 | FDA QSR Compliant
$99.00
$99.00
Lot Inspection Procedure | ISO 13485 | FDA QSR Compliant
$119.00
$119.00

Technology Transfer Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00

Software Validation Procedure | ISO 13485 | FDA QSR Compliant
$149.00
$149.00
ISO 13485 Label Review and Approval Procedure
$139.00
$139.00

ISO 13485 Contract Review Procedure
$49.00
$49.00

CE Marking Procedure
$149.00
$149.00

Post Market Surveillance Procedure
$149.00
$149.00

Software as a Medical Device (SaMD) Development Procedure
$149.00
$149.00

Software Clinical Evaluation Procedure
$149.00
$149.00

Cleanroom Design and Validation Procedure
$149.00
$149.00

Customer Requirements Validation Procedure
$149.00
$149.00

Device History Record Procedure
$149.00
$149.00

Periodic Safety Update Report (PSUR) Procedure
$139.00
$139.00

Failure Investigation Procedure
$149.00
$149.00

Purchase Order Procedure | ISO 13485
$99.00
$99.00

Medical Device Product Performance Specification Procedure
$149.00
$149.00

ISO 13485 Process Validation Procedure Bundle
$399.00
$399.00

Medical Device Process Validation Procedure
$149.00
$149.00
Environmental Controls Procedure | ISO 13485 | FDA QSR Compliant
$119.00
$119.00
Medical Device Clinical Investigation Plan
$49.00
$49.00
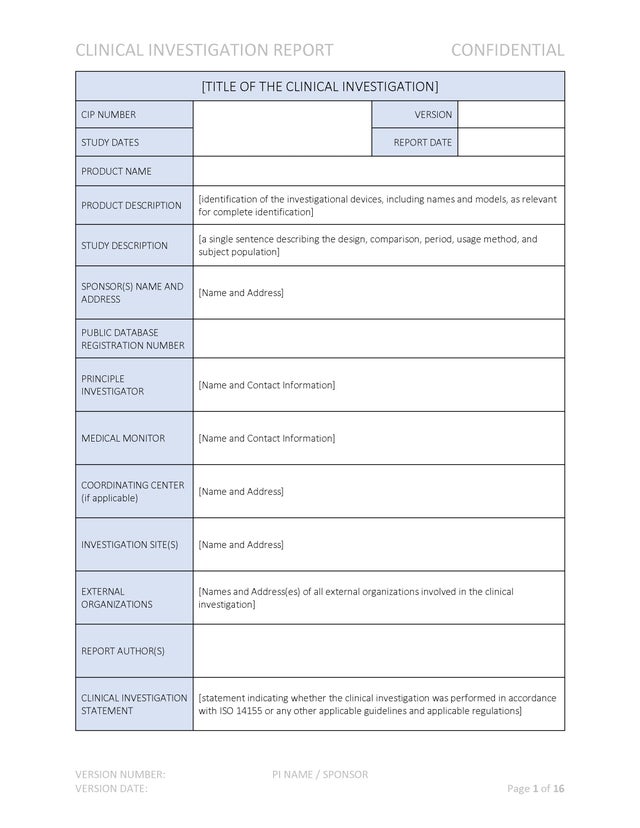
Medical Device Clinical Investigation Report
$49.00
$49.00