- STORE
- >
- ISO 13485 QUALITY SYSTEM PROCEDURES
- >
- ISO 13485 PRODUCT REALIZATION PROCEDURES
- >
- ISO 13485 PRODUCTION SERVICE PROVISION PROCEDURES
- >
- Lot Inspection Procedure | ISO 13485 | FDA QSR Compliant
Lot Inspection Procedure | ISO 13485 | FDA QSR Compliant
$119.00
$119.00
Unavailable
per item
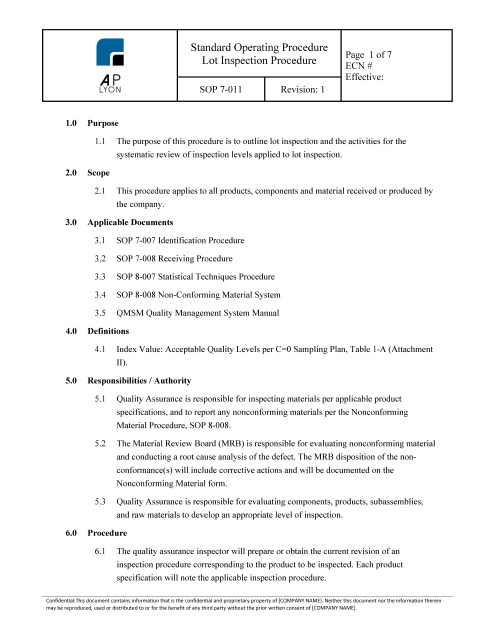
The ISO 13485 Lot Inspection Procedure governs lot inspection and the systematic review of inspection levels applied to lot inspection.
- ISO 13485:2016
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
SKU:
SOP 7-011
Lot Inspection ProcedureThe ISO 13485 Lot Inspection Procedure governs lot inspection and the systematic review of inspection levels applied to lot inspection.
ISO 13485 Lot Inspection Procedure OverviewThe Lot Inspection Procedure applies to all products, components and material received or produced by the company.
The Lot Inspection Procedure includes an quality record form template and a lot inspection data form template. ISO 13485 Lot Inspection Procedure ComplianceThe ISO 13485 Lot Inspection Procedure is FDA QSR and ISO 13485:2016 compliant.
To learn more about our ISO 13485 Lot Inspection Procedure contact us at [email protected]
RELATED LINKSMedical Device Quality Systems
Nonconforming Materials Procedure Identification Procedure Statistical Techniques Procedure Medical Device Quality Management System BASE Medical Device Quality Management System BASE PLUS Medical Device Quality Management System MANUFACTURING Medical Device Quality Management System MANUFACTURING PLUS |
|
Customer's also viewed
Identification Procedure | ISO 13485 | FDA QSR Compliant
$99.00
The ISO 13485 Identification Procedure governs the identification and the acceptance status of finished medical devices, components, materials, in-process devices, and returned devices manufactured by the company.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
Nonconforming Material Procedure | ISO 13485 | FDA QSR Compliant
$149.00
The ISO 13485 Nonconforming Material Procedure establishes a process to control and disposition nonconforming material identified during receiving, in-process, or final product acceptance activity.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- Includes Related Forms
- MS Word Format
- Digital Content - Instant Download
Statistical Techniques Procedure | ISO 13485 | FDA QSR Compliant
$149.00
The ISO 13485 Statistical Techniques Procedure provides guidance on statistical analysis methods used to support medical device sampling plans, failure analysis and validation data analysis.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
Device History Record Procedure
$149.00
The Device History Record Procedure governs the creation of a Device History Record (DHR) of a finished device or critical component for each work order and establishes the process for final release into finished goods.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- Includes Related Forms
- MS Word Format
- Digital Content - Instant Download