- STORE
- >
- ISO 13485 QUALITY SYSTEM PROCEDURES
- >
- ISO 13485 PRODUCT REALIZATION PROCEDURES
- >
- ISO 13485 PRODUCTION SERVICE PROVISION PROCEDURES
- >
- ISO 13485 Process Validation Procedure Bundle
ISO 13485 Process Validation Procedure Bundle
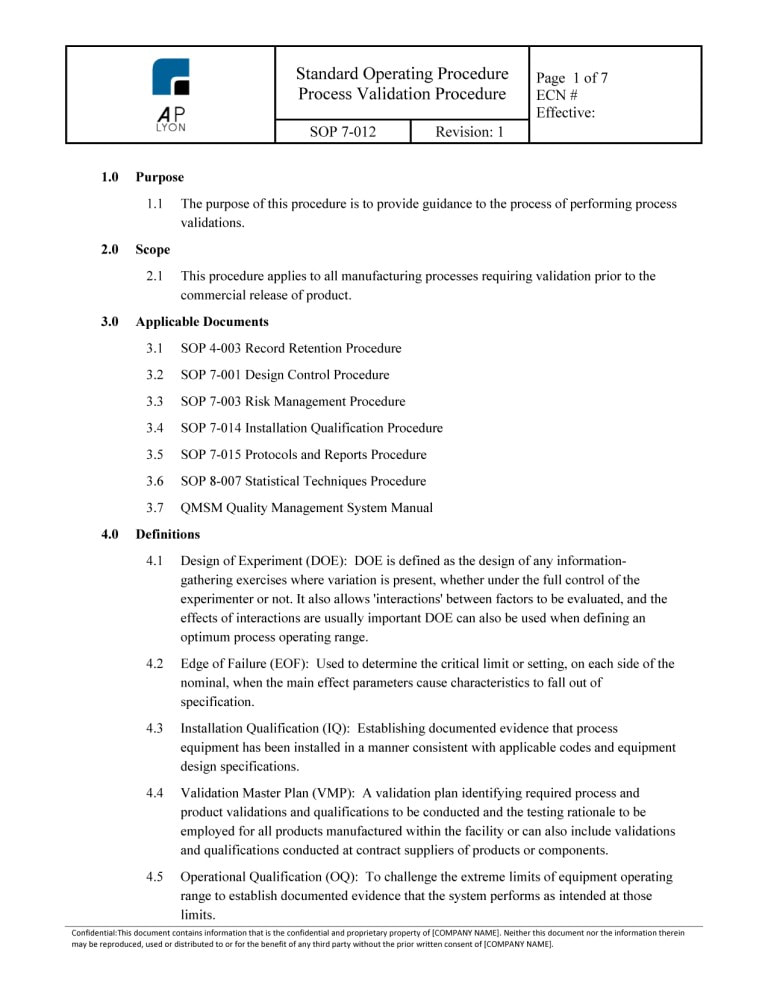
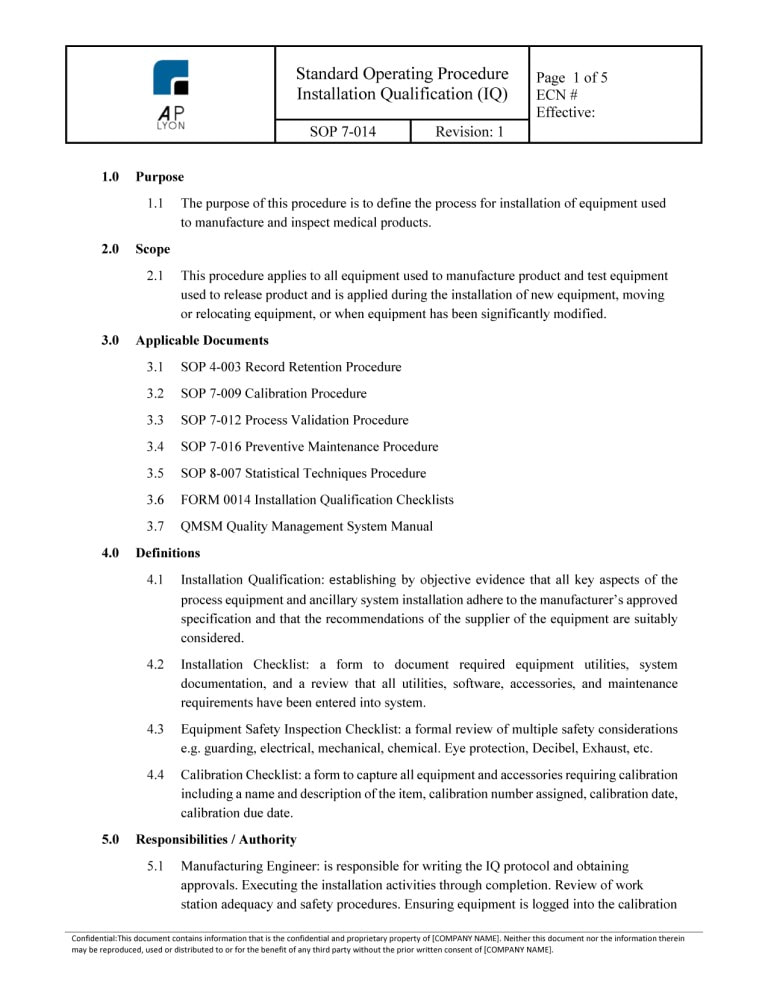
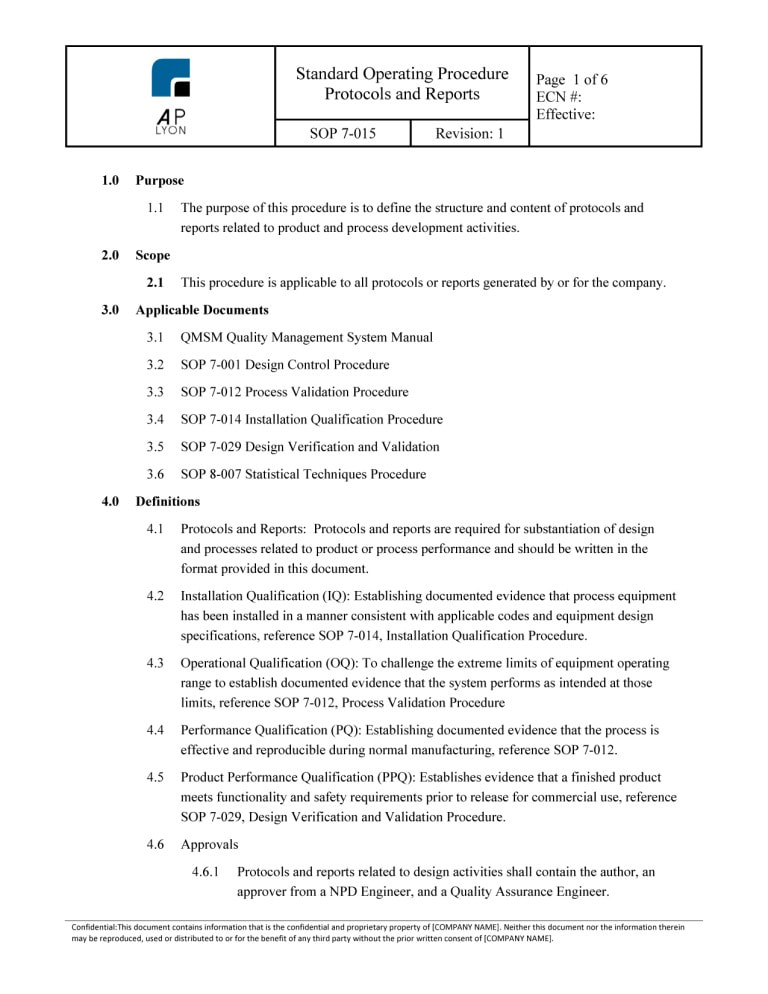
The ISO 13485 Process Validation Procedure Bundle includes 4 procedures related to the validation of medical device processes. The process validation procedures are ISO 13485:2016 and FDA QSR compliant.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Includes Related Form(s)
- Digital Content - Instant Download
ISO 13485 Process Validation Procedure BundleThe Process Validation Procedure Bundle includes four (4) ISO 13485:2016 compliant procedures related to medical device process validation.
Process Validation Procedure Bundle - Overview
Process Validation Procedure Bundle - ComplianceThe Process Validation Procedure Bundle content is ISO 13485:2016 and FDA QSR Compliant.
To learn more about our ISO 13485 Process Validation Procedure Bundle contact us at [email protected]
|
Process Validation Procedure Bundle Content
|
RELATED LINKS
Risk Management Procedure Bundle
Clinical Evaluation Procedure Bundle
Complaint Handling Procedure Bundle
Medical Device Labeling Procedure Bundle
Medical Device Software Procedure Bundle
Document Control Procedure Bundle
Cleanroom Procedure Bundle
Customer's Also Viewed
Medical Device Process Validation Procedure
The Medical Device Process Validation Procedure provides guidance on medical device process validations. The ISO 13485Process Validation Procedure applies to all medical device manufacturing processes requiring validation prior to the commercial release of product.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
Statistical Techniques Procedure | ISO 13485 | FDA QSR Compliant
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
Medical Device Quality Management System | MANUFACTURING
The Medical Device Quality Management System | MANUFACTURING product is designed for companies engaged in both product development and the manufacture of medical devices. The system is rich in content and provides detailed instruction governing research and development, manufacturing and post commercialization activities. The system is configured for companies desiring ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 60 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Quality Management System | MANUFACTURING PLUS
The Medical Device Quality Management System | MANUFACTURING PLUS System is our "top line" QMS product configured for companies engaged in the design and manufacture of medical devices seeking ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 72 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download