- STORE
- >
- ISO 13485 PROCEDURE BUNDLES
- >
- Clinical Evaluation Procedure Bundle
Clinical Evaluation Procedure Bundle
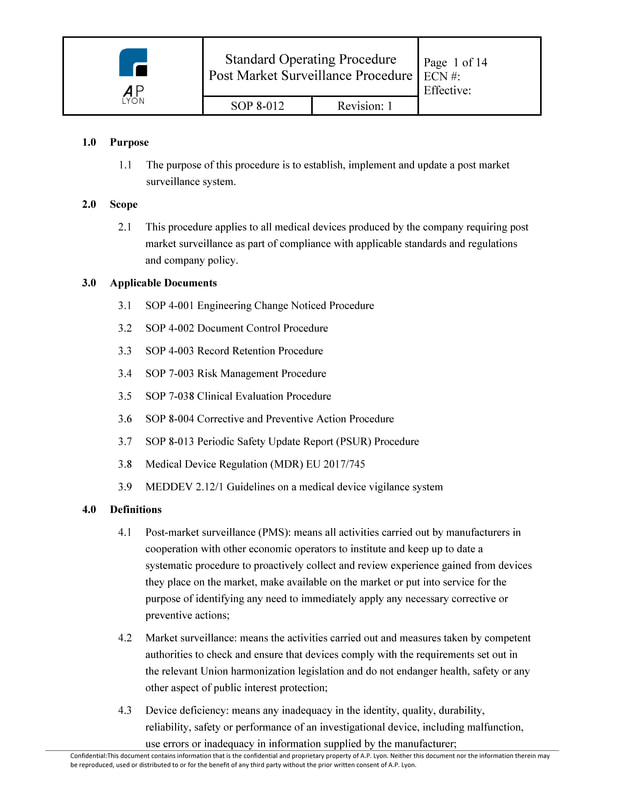
The Clinical Evaluation Procedure Bundle includes Standard Operating Procedures and Forms relating to Clinical Evaluations, Post Market Clinical Follow-Up, Post Market Surveillance and Clinical Investigations.
- MDR EU 2017/745 Compliant
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MEDDEV 2.7.1, Rev 4
- MS Word Format
- Instant Download Digital Content
Clinical Evaluation Procedure BundleThe Clinical Evaluation Procedure Bundle is a great value product that includes Standard Operating Procedures and templates relating to Clinical Evaluations, Post Market Clinical Follow-Up, Post Market Surveillance and CE Marking.
Clinical Evaluation Procedure Bundle ContentsThe Clinical Evaluation Procedure Bundle includes the following procedures:
1) Clinical Evaluation ProcedureThe Clinical Evaluation Procedure governs the entire clinical evaluation process from scope definition through Clinical Evaluation Report (CER). The MEDDEV 2.7/1 Rev 4 compliant Clinical Evaluation Procedure also governs Post Market Clinical Follow-up (PMCF).
2) Post Market Clinical Follow-Up (PMCF) PlanPost Market Clinical Follow-up (PMCF) Plan. User friendly, detailed guidance, MDR 2017/745 format. The purpose of the PMCF Plan is to guide users in complying with the requirements of the MDR with respect to the compilation of the PMCF plan.
3) Post Market Clinical Follow-Up (PMCF) Evaluation ReportThe PMCF Evaluation Report template guides manufacturers in complying with the requirements of the MDR with respect to the compilation of the PMCF evaluation report.
4) Post Market Surveillance ProcedureThe Post Market Surveillance Procedure governs all post market surveillance activities and provides instruction on: Post Market Surveillance Plan and Report (Includes Sample Plan); Post Market Clinical Follow-Up (PMCF) requirements; and Periodic Safety Update Reports (PSUR) content when applicable.
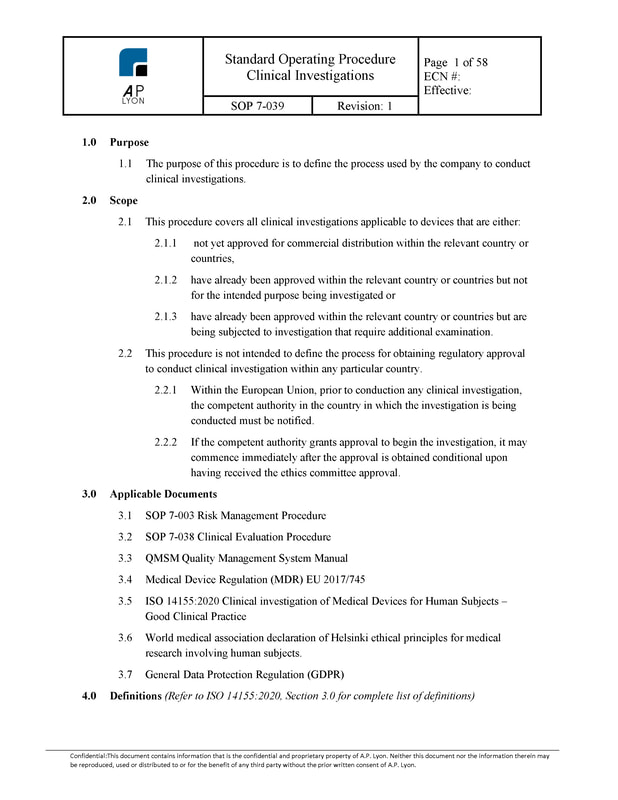
5) Clinical Investigation ProcedureThe Clinical Investigation Procedure complies with ISO 14155:2020. The Clinical Investigation procedure includes detailed information on all aspects of clinical investigations. The product includes a CIP protocol and report template, Investigators' brochure (IB) template, investigation site initiation and monitoring forms, informed consent template and guidance on CRF construction.
Clinical Evaluation Procedure Bundle ComplianceThe Clinical Evaluation Procedure Bundle is MEDDEV 2.7.1 Rev 4, ISO 14155:2020, MDR 2017/745, FDA QSR and ISO 13485:2016 compliant.
For more information about our Clinical Evaluation Procedure Bundle contact us at [email protected]
|
Clinical Evaluation Bundle Contents |
Customer's also viewed
Clinical Investigation Procedure
- ISO 14155:2020 Compliant
- MDR EU 2017/745 Compliant
- ISO 13485:2016 Compliant
- Includes Multiple Forms
- MS Word Format
- Instant Download Digital File
Clinical Evaluation Procedure
The Clinical Evaluation Procedure governs the entire clinical evaluation process from scope definition through Clinical Evaluation Report (CER).The MEDDEV 2.7/1 Rev 4 compliant Clinical Evaluation Procedure also governs Post Market Clinical Follow-up (PMCF).
- MEDDEV 2.7/1 Rev 4 Compliant
- MDR (EU) 2017/745 Compliant
- ISO 13485:2016 Compliant
- MS Word Format
- Instant Download Digital Content
Post Market Surveillance Procedure
The Post Market Surveillance Procedure governs all post market surveillance activities and provides instruction on: Post Market Surveillance Plans and Reports; and Post Market Clinical Follow-Up (PMCF) requirements including easy to follow templates.
- MEDDEV 2.12/1 Compliant
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MDR EU 2017/745 Compliant
- MS Word Format
- Instant Download Digital Content
Medical Device Quality Management System | MANUFACTURING PLUS
The Medical Device Quality Management System | MANUFACTURING PLUS System is our "top line" QMS product configured for companies engaged in the design and manufacture of medical devices seeking ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 72 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download