- STORE
- >
- ISO 13485 PROCEDURE BUNDLES
- >
- Risk Management Procedures Bundle | ISO 13485 | FDA QSR Compliant
Risk Management Procedures Bundle | ISO 13485 | FDA QSR Compliant
The Risk Management Procedure Bundle includes four (4) ISO 14971:2019 compliant standard operating procedures relating to the risk management process for medical devices.
- EN ISO 14971:2019 Compliant
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Includes Related Forms
- MS Word and Excel Format
- Digital Content - Instant Download
Risk Management Procedure BundleThe Risk Management Procedure Bundle includes four (4) Standard Operating Procedures and related Forms encapsulating the entire risk management process from product development through post-market feedback.
Risk Management Procedure Bundle - ContentThe Risk Management Procedure Bundle includes the following risk management related procedures:
1) Risk Management ProcedureThe Risk Management Procedure serves as the top level procedure governing design and production risk management, development of risk management plans, reports, and risk management files. The procedure includes multiple forms complete with helpful instruction.
2) Design Risk Management ProcedureThe Design Risk Management Procedure provides detailed step by step guidance to help design teams properly identify design risks associated with the development of a medical device. The procedure includes multiple forms to capture the entire process in Excel format.
3) Process Risk Management ProcedureThe Process Risk Management Procedure provides detailed step by step guidance to help design teams properly identify process risks associated with the production of a medical device. The procedure includes multiple forms to capture the entire process in Excel format.
4) Design Analysis ProcedureThe Design Analysis Procedure includes detailed guidance on how to conduct DFMECA, PFMECA, House of Quality(HOQ) and Fault Tree Analysis(FTA). The procedure includes all related forms and ground rules for each study,
Risk Management Procedure Bundle ComplianceThe Risk Management Procedures comply with the requirements of ISO 14971:2019, ISO / TR 24971, ISO 13485:2016, and FDA 21 CFR Part 820.
For more information about our Risk Management Procedure Bundle contact us at [email protected]
|
Risk Management Bundle Contents |
Customer's also viewed
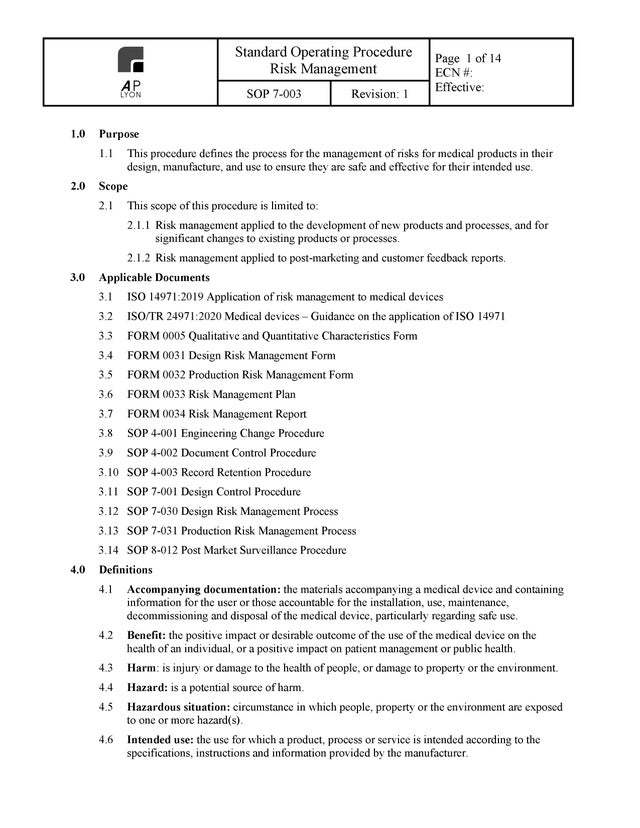
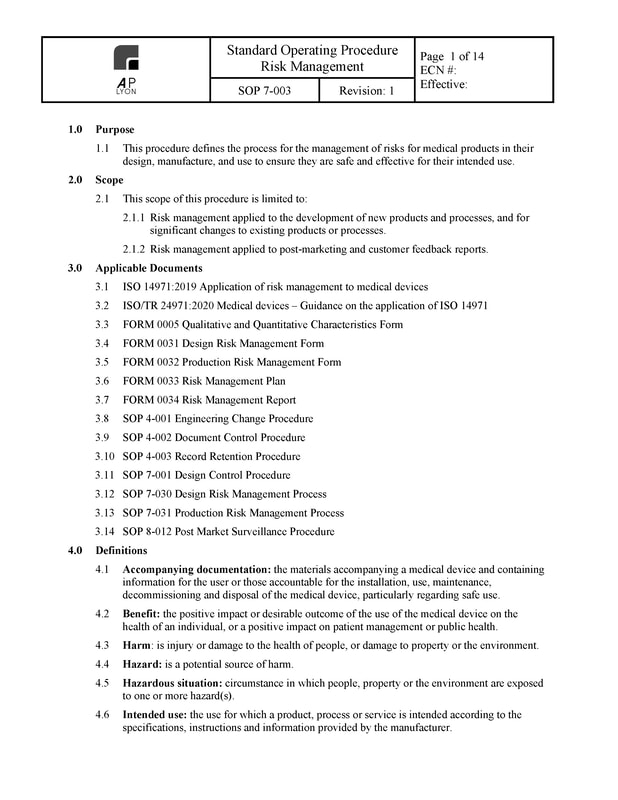
Risk Management Procedure
The ISO 14971:2019 compliant Risk Management Procedure defines the process for the management of risks for medical products in their design, manufacture, and use to ensure they are safe and effective for their intended use.
- ISO 14971:2019 Compliant
- FDA QSR Compliant
- ISO 13485:2016 Compliant
- MDR EU 2017/745 Compliant
- Includes Related Forms
- MS Word and Excel Format
- Digital Content - Instant Download
Medical Device Quality Management System | BASE PLUS SYSTEM
The Medical Device Quality Management System | BASE PLUS is configured for companies engaged in the design and manufacture of medical devices who need only the minimum required content to obtain ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 38 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Quality Management System | DESIGN PLUS
The DESIGN PLUS Quality Management System (QMS) is configured for companies engaged in research and development of medical devices but perform no manufacturing (Specification Developers). The Design Plus QMS system is configured for companies desiring ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 46 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Quality Management System | MANUFACTURING PLUS
The Medical Device Quality Management System | MANUFACTURING PLUS System is our "top line" QMS product configured for companies engaged in the design and manufacture of medical devices seeking ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 72 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download