- STORE
- >
- ISO 13485 QUALITY SYSTEM PROCEDURES
- >
- ISO 13485 PRODUCT REALIZATION PROCEDURES
- >
- ISO 13485 PRODUCTION SERVICE PROVISION PROCEDURES
- >
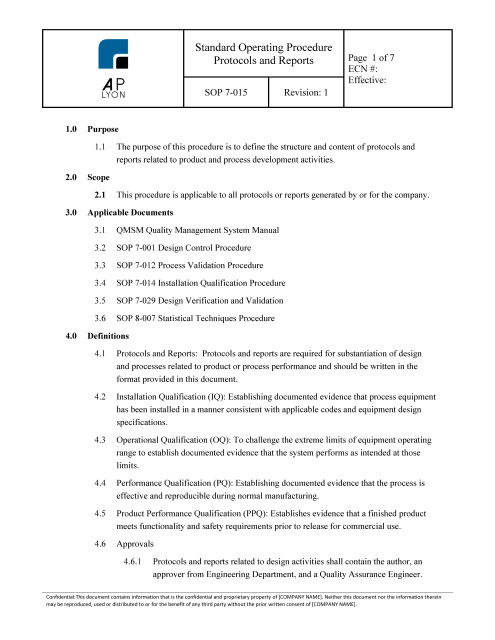
- Validation Protocol and Report Procedure | ISO 13485 | FDA QSR Compliant
Validation Protocol and Report Procedure | ISO 13485 | FDA QSR Compliant
$99.00
$99.00
Unavailable
per item
The ISO 13485 Validation Protocol and Report Procedure governs the structure and content of all validation protocols and reports (IQ, OQ, PQ and PPQ) made part of product development and process validation.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
SKU:
SOP 7-015
ISO 13485 Protocol and Report ProcedureThe Validation Protocol and Report Procedure governs the structure and content of protocols and reports related to equipment, process and product.
ISO 13485 Protocols and Reports Procedure OverviewThe Validation Protocol and Report Procedure instructs on the development of Installation Qualification (IQ) Protocols, Operational Qualification (OQ) Protocols, Performance Qualification (PQ) Protocols and Product Performance Qualification (PPQ) Protocols.
The Validation Protocol and Report Procedure also provides instruction on the format and required content of all validation final reports. ISO 13485 Protocol and Report Procedure ComplianceThe Validation Protocol and Report Procedure is FDA QSR and ISO 13485:2016 compliant.
To learn more about our ISO 13485 Validation Protocol and Report Procedure contact us at [email protected]
RELATED LINKS |
|
Customer's also viewed
ISO 13485 Process Validation Procedure Bundle
$399.00
The ISO 13485 Process Validation Procedure Bundle includes 4 procedures related to the validation of medical device processes. The process validation procedures are ISO 13485:2016 and FDA QSR compliant.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Includes Related Form(s)
- Digital Content - Instant Download
Statistical Techniques Procedure | ISO 13485 | FDA QSR Compliant
$149.00
The ISO 13485 Statistical Techniques Procedure provides guidance on statistical analysis methods used to support medical device sampling plans, failure analysis and validation data analysis.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
Installation Qualification IQ Procedure | ISO 13485 | FDA QSR Compliant
$119.00
The ISO 13485 Installation Qualification (IQ) Procedure governs the installation of equipment used to manufacture medical devices and all test equipment used to release product.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- Includes Related Forms
- MS Word Format
- Digital Content - Instant Download
Design Verification - Validation Procedure | ISO 13485 | FDA QSR Compliant
$149.00
The ISO 13485 Design Verification and Validation Procedure defines governs design verification and validation activity being conducted as part of medical device development cycle.
- ISO 13485 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download