- STORE
- >
- ISO 13485 QUALITY SYSTEM PROCEDURES
- >
- ISO 13485 PRODUCT REALIZATION PROCEDURES
- >
- ISO 13485 PRODUCTION SERVICE PROVISION PROCEDURES
- >
- Sterile Load Preparation - Release Procedure | ISO 13485 | FDA QSR Compliant
Sterile Load Preparation - Release Procedure | ISO 13485 | FDA QSR Compliant
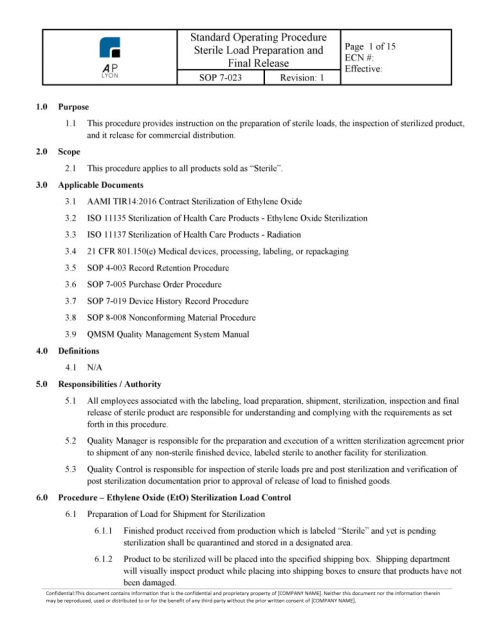
The ISO 13485 Sterile Load Preparation and Release Procedure provides instruction preparing sterile loads, inspection of loads pre and post sterilization, and the requirements to release sterile loads for commercial distribution.
- ISO 13485:2016 Compliant
- CFR 801.150 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
ISO 13485 Sterile Load Preparation and Release ProcedureThe Sterile Load Preparation and Release Procedure governs the preparation and organization of a sterile load, the inspection of post-sterilized product, and the final release of a sterile load for commercial distribution.
Sterile Load Preparation and Release Procedure OverviewThe Sterile Load Preparation and Release Procedure includes everything you need to organize, process and accept sterile loads within your facility for multiple sterilization methods (e.g. EO, Gamma).
The procedure includes: sterile load data forms to properly organize a sterile load; sterile load labeling for each stage of the process to ensure load control; and includes the sterilization records (post sterilization) required for the final release of a sterile load to finished goods.
Sterile Load Preparation and Release Procedure ComplianceThe Sterile Load Preparation and Release Procedure is ISO 13485:2016 and FDA QSR Compliant.
To learn more about our Sterile Load Preparation and Release Procedure contact us at [email protected]
RELATED LINKS |
|
Customer's also viewed
Medical Device Sterilization Procedure Bundle
- ISO 13485:2016 Compliant
- ISO 1135 Compliant
- ISO 11137 Compliant
- 21 CFR Part 820 Compliant
- MS Word Format
- Digital Product - Instant Download
Medical Device Quality Management System | BASE PLUS SYSTEM
The Medical Device Quality Management System | BASE PLUS is configured for companies engaged in the design and manufacture of medical devices who need only the minimum required content to obtain ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 38 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Quality Management System | MANUFACTURING
The Medical Device Quality Management System | MANUFACTURING product is designed for companies engaged in both product development and the manufacture of medical devices. The system is rich in content and provides detailed instruction governing research and development, manufacturing and post commercialization activities. The system is configured for companies desiring ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 60 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Quality Management System | MANUFACTURING PLUS
The Medical Device Quality Management System | MANUFACTURING PLUS System is our "top line" QMS product configured for companies engaged in the design and manufacture of medical devices seeking ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 72 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download