- STORE
- >
- ISO 13485 QUALITY SYSTEM PROCEDURES
- >
- ISO 13485 PRODUCT REALIZATION PROCEDURES
- >
- Medical Device Clinical Investigation Report
Medical Device Clinical Investigation Report
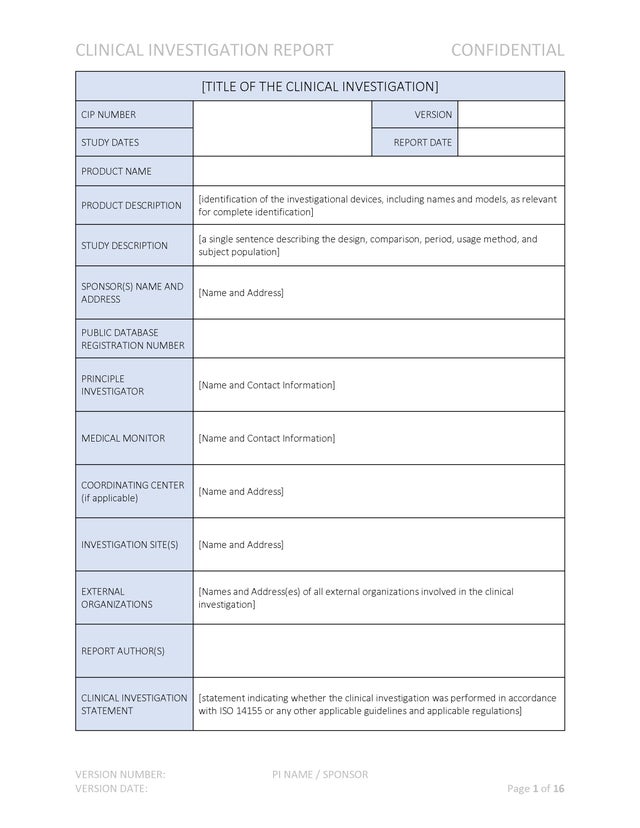
The Clinical Investigation Report complies with the requirements of ISO 14155:2020. The report provides an excellent structure for summarizing the clinical investigation plan, communicating results of the investigation and overall conclusions.
- ISO 14155:2020 Compliant
- MDR 2017/745 Compliant
- MS Word Format
- Instant Download - Digital Content
Clinical Investigation ReportThe Clinical Investigation Report complies with the requirements of ISO 14155:2020. The report provides an excellent structure for summarizing the clinical investigation plan, communicating results of the investigation and overall conclusions.
Clinical Investigation Report - ComplianceThe Clinical Investigation Report complies with MDR 2017/745 and ISO 14155:2020
|
|
Customer's also viewed
Clinical Investigation Procedure
- ISO 14155:2020 Compliant
- MDR EU 2017/745 Compliant
- ISO 13485:2016 Compliant
- Includes Multiple Forms
- MS Word Format
- Instant Download Digital File
Clinical Evaluation Procedure
The Clinical Evaluation Procedure governs the entire clinical evaluation process from scope definition through Clinical Evaluation Report (CER).The MEDDEV 2.7/1 Rev 4 compliant Clinical Evaluation Procedure also governs Post Market Clinical Follow-up (PMCF).
- MEDDEV 2.7/1 Rev 4 Compliant
- MDR (EU) 2017/745 Compliant
- ISO 13485:2016 Compliant
- MS Word Format
- Instant Download Digital Content
Medical Device Clinical Investigation Plan
The Clinical Investigation Plan (CIP) complies with ISO 14155:2020. Robust 28 page user friendly form addresses each required clinical investigation plan element defined in ISO 14155 and MDR 2017/745.
- ISO 14155:2020 Compliant
- MDR 2017/745 Compliant
- MS Word Format
- Instant Download - Digital Product
Medical Device Quality Management System | MANUFACTURING PLUS
The Medical Device Quality Management System | MANUFACTURING PLUS System is our "top line" QMS product configured for companies engaged in the design and manufacture of medical devices seeking ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 72 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download