- STORE
- >
- ISO 13485 QUALITY SYSTEMS
- >
- Medical Device Design and Development Procedures
Medical Device Design and Development Procedures
The Medical Device Design and Development Procedures product contains multiple procedures related to the design and development of medical devices in accordance with FDA QSR and ISO 13485:2016 design and development requirements. This product is intended for companies needing procedures to augment their existing QMS to include design control and related content. All our procedures are ISO 13485:2016 and FDA QSR compliant.Quality Management System Manual
- 27 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Design and Development Procedures
Design and Development Procedures - Benefits
Design and Development Procedures - Compliance
|
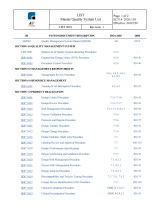
Download the QMS Content List with References to ISO 13485 Clauses and FDA QSR Sections Here:
|
COMPARE THE CONTENT OF ALL
OF OUR QMS PRODUCTS | ||||||||
Frequently Asked Questions
|
How long does it take to install the Design and Development Procedures?
The DESIGN quality management system (QMS) procedures can be implemented in as little as 1-2 weeks. The major advantage of our procedures is 85% of the work is already completed upon purchase. The remaining 15% is expended in aligning our procedures with your unique organizational structure, and procedure training. Can the content be integrated with EQMS software? Yes! Many of our customers are using our products to either establish or augment their EQMS content. The procedures can be easily aligned with EQMS work instructions, workflows and forms. Does A.P. Lyon provide support with procedure installation? Absolutely! Many of our clients will leverage our resources to either install our procedures or ask us to audit their quality management system (QMS) post installation. Are the Design and Development Procedures ISO 13485 and FDA QSR Compliant? Yes! These procedures are both ISO 13485:2016 and FDA compliant. Have the Procedures been audited? Yes! Our Design and Development Procedures have been successfully audited by notified bodies, FDA, and been challenged by numerous third party audits. |
DO THE MATH
When you consider the hours required to develop a system internally or the cost associated with hiring an outside consultant to develop a quality management system for you we trust that you will see the value of our product offerings.
PRODUCT TESTIMONIALS
/
I have purchased several of your products and I have always been happy with the quality of your work. Thanks for providing a great product.
Karen Atwood
|
|
|
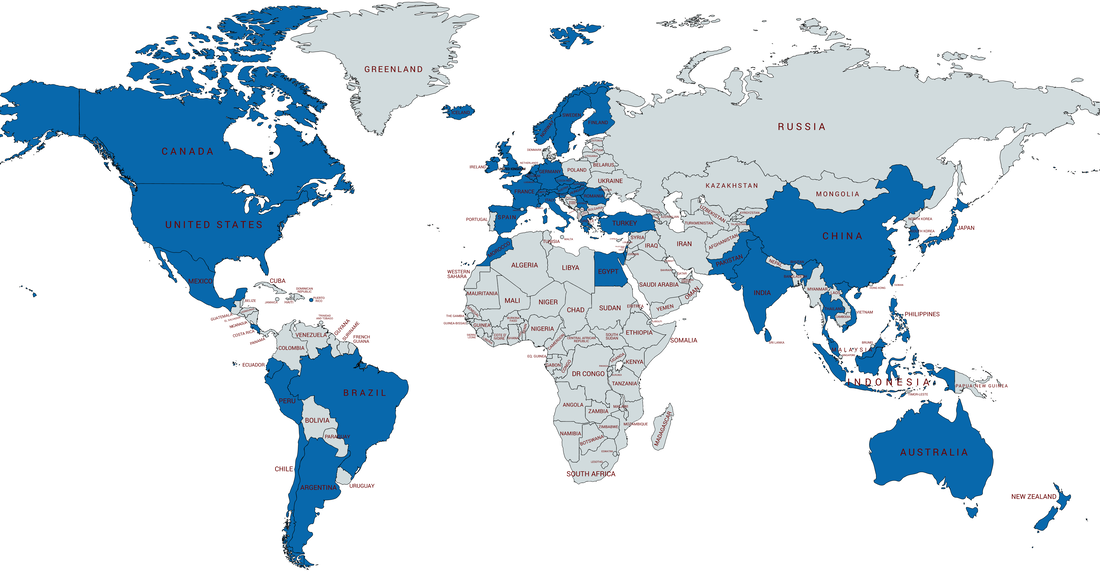
Map of Countries Served |
RELATED LINKS
Medical Device Quality Management System BASE
Medical Device Quality Management System BASE PLUS
Medical Device Quality Management System DESIGN PLUS
Medical Device Quality Management System MANUFACTURING
Medical Device Quality Management System MANUFACTURING PLUS
Customer's also viewed
Medical Device Quality Management System | BASE SYSTEM
The ISO 13485 BASE Quality Management System (QMS) product contains procedures that serve as the foundation for your QMS needs. ISO 13485 and FDA QSR compliant. Products installed in 46 countries and growing!
- Quality Management System Manual
- 23 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Quality Management System | BASE PLUS SYSTEM
The Medical Device Quality Management System | BASE PLUS is configured for companies engaged in the design and manufacture of medical devices who need only the minimum required content to obtain ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 38 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Quality Management System | DESIGN PLUS
The DESIGN PLUS Quality Management System (QMS) is configured for companies engaged in research and development of medical devices but perform no manufacturing (Specification Developers). The Design Plus QMS system is configured for companies desiring ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 46 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Quality Management System | MANUFACTURING
The Medical Device Quality Management System | MANUFACTURING product is designed for companies engaged in both product development and the manufacture of medical devices. The system is rich in content and provides detailed instruction governing research and development, manufacturing and post commercialization activities. The system is configured for companies desiring ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 60 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download
Medical Device Quality Management System | MANUFACTURING PLUS
The Medical Device Quality Management System | MANUFACTURING PLUS System is our "top line" QMS product configured for companies engaged in the design and manufacture of medical devices seeking ISO 13485:2016 certification and FDA QSR compliance. Products installed in 46 countries and growing!
- Quality Management System Manual
- 72 Procedures and Related Forms (MS Word and Excel)
- ISO 13485:2016 and FDA QSR Compliant
- MDR EU 2017/745 Compliant
- Digital Content - Instant Download