- STORE
- >
- ISO 13485 QUALITY SYSTEM PROCEDURES
- >
- ISO 13485 RESOURCE MANAGEMENT PROCEDURES
- >
- Facility Inspection Procedure
Facility Inspection Procedure
$149.00
$149.00
Unavailable
per item
The ISO 13485 Facility Inspection Procedure defines the process and responsibilities for handling facility inspections conducted by FDA, Notified Bodies, Government Agencies and other third-party inspections.
- ISO 13485 Compliant
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
SKU:
SOP 6-006
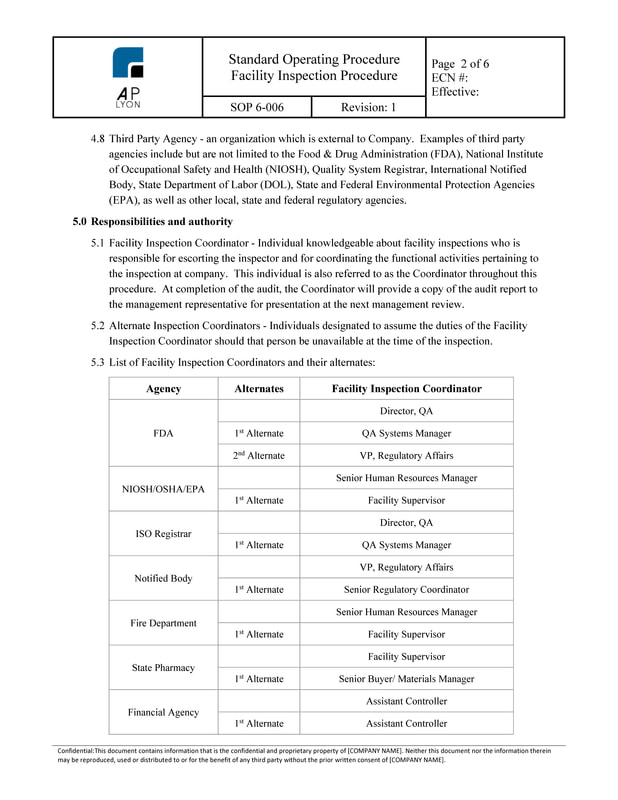
Facility Inspection ProcedureThe Facility Inspection Procedure defines the process and responsibilities for handling facility inspections conducted by FDA, Notified Bodies, Government Agencies and other third-party inspections.
Facility Inspection Procedure - OverviewThe Facility Inspection Procedure identifies assigned inspection personnel and alternates, assigns responsibilities for multiple types of inspections. The Facility Inspection Procedure provides instruction on inspection restrictions and the proper internal handling of facility inspections and inspectors. The procedure also includes instruction on exit interviews, and timelines for required post inspection activities.
Facility Inspection Procedure - ComplianceThe Facility Inspection Procedure is ISO 13485:2016 and FDA 21 CFR Part 820 compliant.
For more information about our ISO 13485 Facility Inspection Procedure contact us at [email protected]
RELATED LINKS |
Facility Inspection Procedure Preview |
Customer's also viewed
Internal Audit Procedure | ISO 13485 | FDA QSR Compliant
$99.00
The ISO 13485 Internal Audit Procedure defines the process, methods and cycle for conducting internal audits.
- ISO 13485:2016
- FDA QSR Compliant
- MS Word Format
- Digital Content - Instant Download
Management Review Procedure | ISO 13485 | FDA QSR Compliant
$119.00
The ISO 13485 Management Review Procedure governs the management review process, management responsibilities, review inputs and outputs, review schedule and includes standardized forms to support all management review activity.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- Management Review Forms
- MS Word Format
- Digital Content - Instant Download
Training Procedure | ISO 13485 | FDA QSR Compliant
$149.00
The Training Procedure governs the methods used to ensure employees possess the necessary training and skills to adequately perform their particular job function.
- ISO 13485:2016 Compliant
- FDA QSR Compliant
- Includes Training Matrix and Training Records Forms
- MS Word Format
- Digital Content - Instant Download